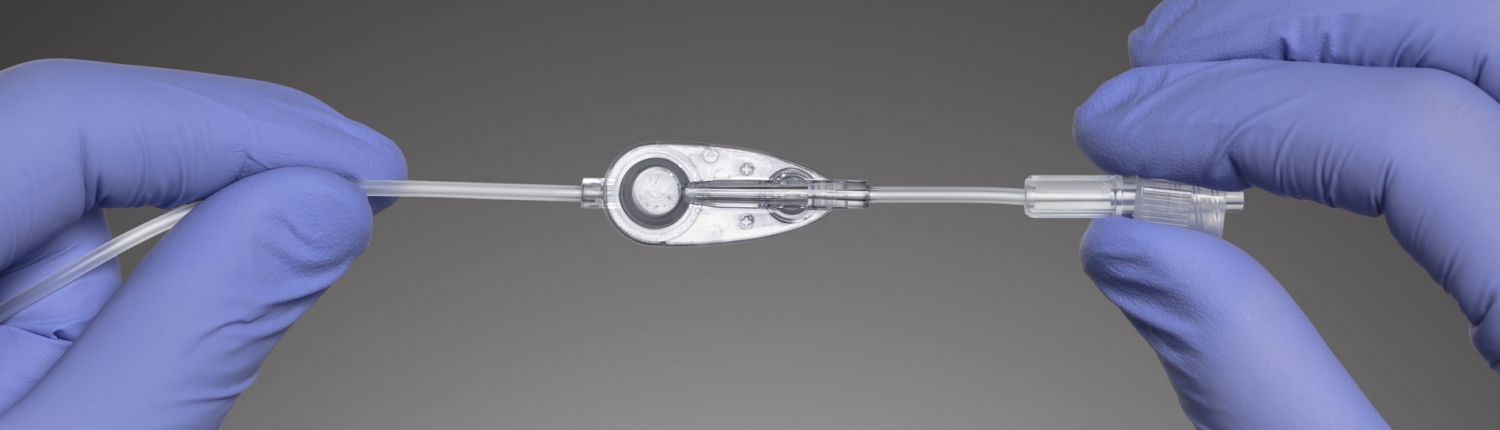
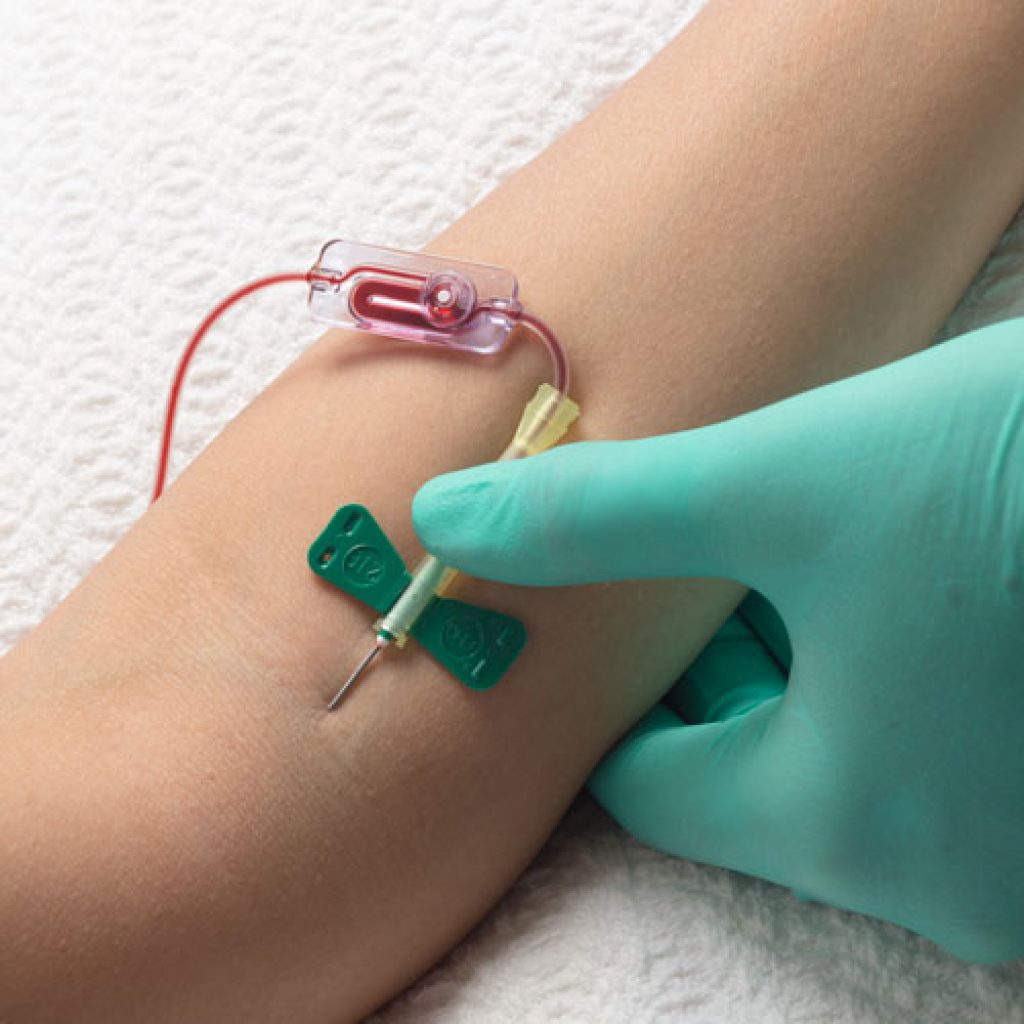
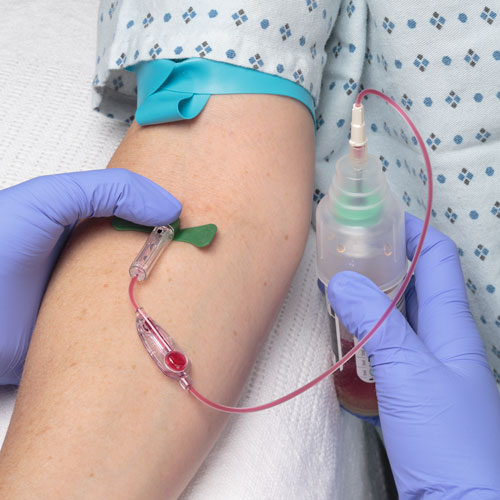
News
- Kurin and Crouse Hospital: A Transformative Partnership in Reducing Contaminated Blood Cultures
- Kurin Works with The Ohio State University Wexner Medical Center on Patient Safety and Increased Access to High Quality Healthcare
- Final Judgment Entered in Kurin’s Favor over Magnolia Medical
- Kurin Announces Appointment of Chief Commercial Officer
- Court Determines that Kurin Does Not Infringe Magnolia Medical Patent
- Kurin, Inc. Reports Record Revenue for Q1 2024
- Kurin, Inc. Files False Advertising Lawsuit Against ICU Medical and Vascular Integrity
- USPTO Examiner Affirms Rejection of Claims Asserted by Magnolia Medical, Inc. against Kurin, Inc.
- USPTO Issues Final Rejection of Magnolia Medical’s ‘483 Patent at Issue in Litigation
- Kurin, Inc. Reports Record Revenue for Q3 2023
{"ticker_effect":"slide-h","autoplay":"true","speed":"4000","font_style":"normal"}
Need more information?
Click here to connect with Kurin.