Francine Touzard Romo, Dianne Auld2, Alison de Abreu, Kimberly Roberts, Gail Jackson, Valerie Whitehead, Emerald O’Rourke, Phinnara Has, Leonard A Mermel. Lifespan Health System, Providence, RI
Background
Lifespan healthcare system implemented the Kurin Lock® ISDD in three acute care hospitals (Rhode Island Hospital [RIH]—a 714-bed tertiary care teaching hospital, The Miriam Hospital [TMH]—a 247-bed community teaching hospital, and Newport Hospital [NH]—a 129-bed community hospital) beginning October 2022 and assessed the impact on Blood culture contamination BCxC and vancomycin utilization in adult EDs, intensive care unit, and step-down units (ICU/SDUs). Blood cultures were obtained by nurses (all 3 hospitals) and phlebotomists (NH) after training by the manufacturer. Blood culture technique was followed per protocol, and growth was monitored using the BioMérieux VIRTUO system. No changes in phlebotomy, laboratory practices, or antimicrobial stewardship interventions were implemented during the study period.
Methods
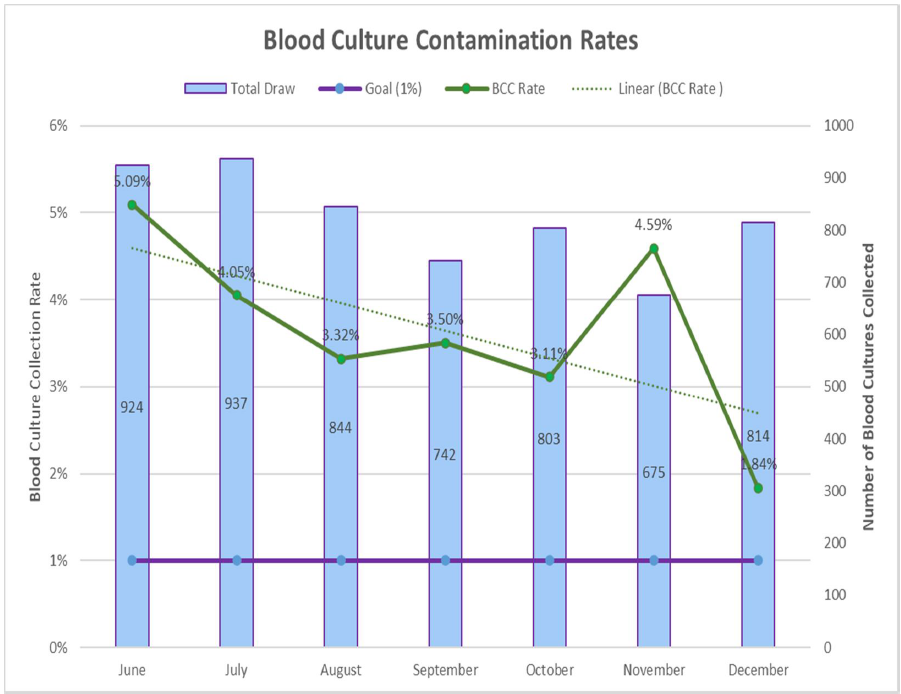
BCxC rates were calculated dividing the number of contaminated cultures (per CDC NHSN commensal list) over the total number of blood cultures/month. The pre-implementation period was defined as the 6 months prior, and the post-implementation period were the months following implementation through December 2023, excluding the month of implementation. Mean BCxC rates prior to Kurin Lock® implementation and after implementation were compared using the Wilcoxon rank sum test. An interrupted time-series analysis was performed using binomial regression models; implementation dates were standardized as “Day 0.” Vancomycin days of therapy (DOT) by order entry indication of “bacteremia” was analyzed using a generalized linear model (Stata/MP 18.0; College Station, TX).
Results
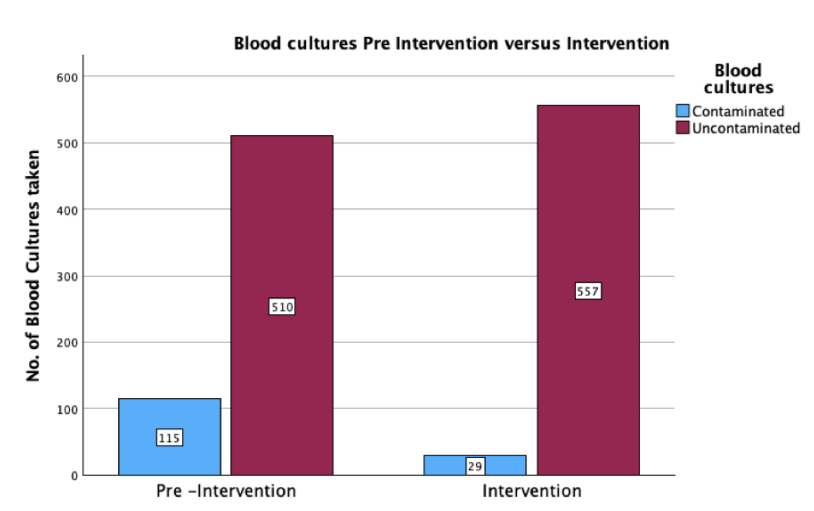
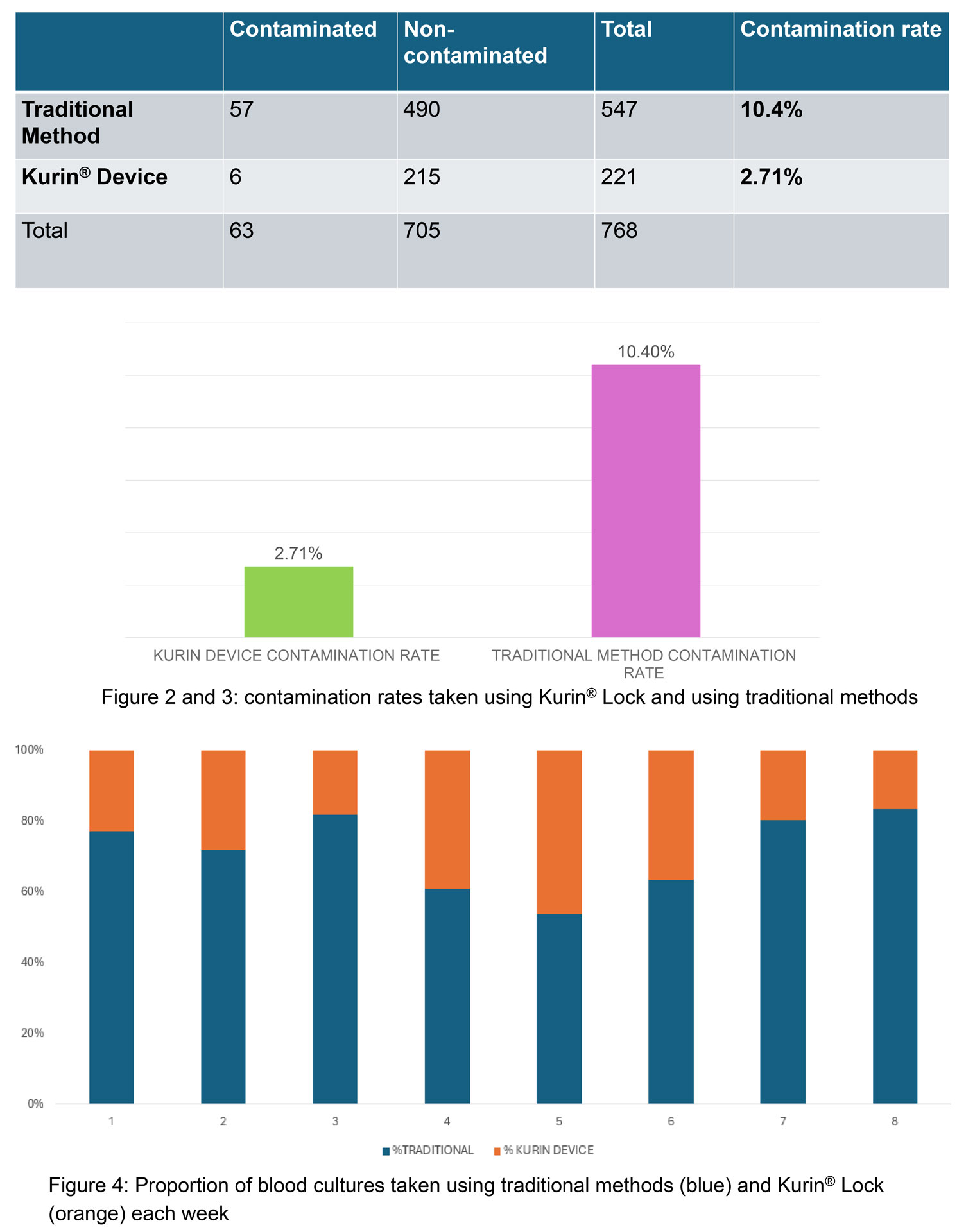
The mean (SD) BCxC rate for all three hospitals and locations declined by 37% [from 3.0% (2.1) to 1.9% (1.4) after the ISDD implementation (P = 0.009)]). The mean number of blood cultures per month was similar during the pre- and post-Kurin period. In time-series analysis, we observed an abrupt 65% decline in BCxC following education and implementation of the Kurin Lock® ISDD at all three hospitals and locations (P = 0.04). Post-Kurin BCxC rates remained lower than pre-Kurin rates at 400 days after implementation; however, increasing BCxC rates were observed post-implementation, particularly in the Eds. TMH and NPH ED BCxC rates were already declining pre-Kurin Lock® implementation. Nonetheless, rates of contamination only reached 1% or less after the ISDD was implemented. The authors did not observe any significant change in mean vancomycin DOT for bacteremia up to 200 days after Kurin implementation in ICU/SDUs.
Conclusion
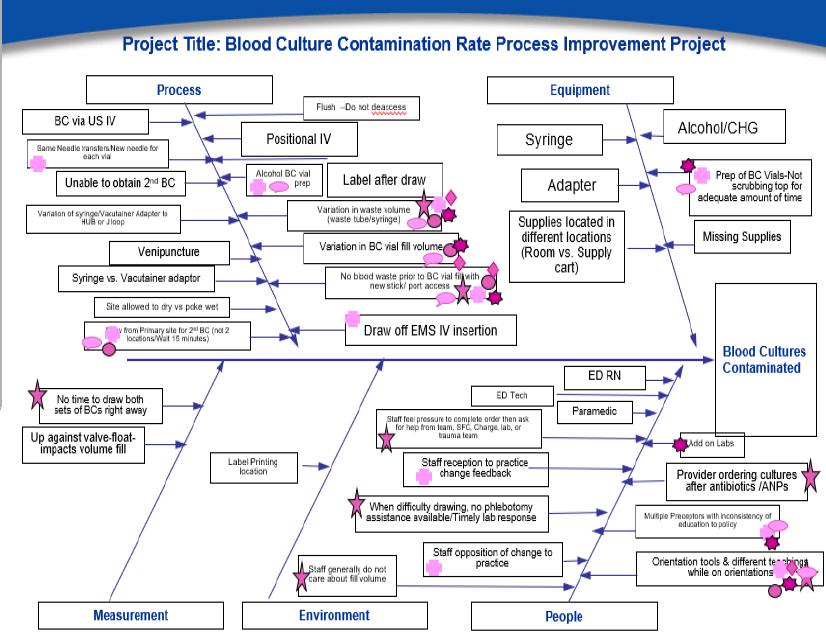
Although lower BCxC rates were sustained with time, the abrupt decline in rates and intermittent spikes resulting in an upward trend highlights the critical value of continuous quality improvement efforts focusing on best practices paired with the device implementation. An independent review of blood culture collection practices by the manufacturer noted: (1) occasional blood draws from existing intravascular catheters; (2) inconsistent skin preparation; (3) placement of blood culture bottles on patient’s beds; (4) inconsistent stocking of supplies; and (5) new staff unawareness of allowing lock side channel blood flow to stop before accessing the blood culture bottles. In addition, ED staff were not utilizing peripheral IV sets with an attached extension (PV-18) designed to reduce contamination from touch points. Based on our findings, some action items we suggest are critical when implementing an ISDD include: hands-on education when onboarding new staff, emphasizing the importance of drawing blood from fresh venipuncture and not from previously inserted peripheral IVs, establishing PAR levels for all blood culture collection items, stocking enough supplies in IV carts, and provide targeted feedback to individual staff associated with high levels of blood culture contamination.
Implementation of the Kurin Lock® ISDD lowered BCxC in large academic and community hospital settings. Continuous quality improvement efforts regarding best practices for blood collection through skill development and staff accountability are important to assure the efficacy and cost-effectiveness of this intervention.
Read full text here.