Analysis of the Clinical Significance of Positive Blood Cultures in the Emergency Department: A Single-Center Study
Karlath FA, Rehan MA, Geigor A, Mitchell J, Arnaout S, Greenough TC, and Elliso RT.
UMass Memorial Health
Open Forum Infectious Disease June 17, 2025
Background
There have been major advances in blood culture technology in the last decade with both faster and more sensitive pathogen detection as well as more precise species identification. We have performed a reassessment of the results of positive blood cultures in this new clinical microbiology era with a focus on contaminant identification.
Methods
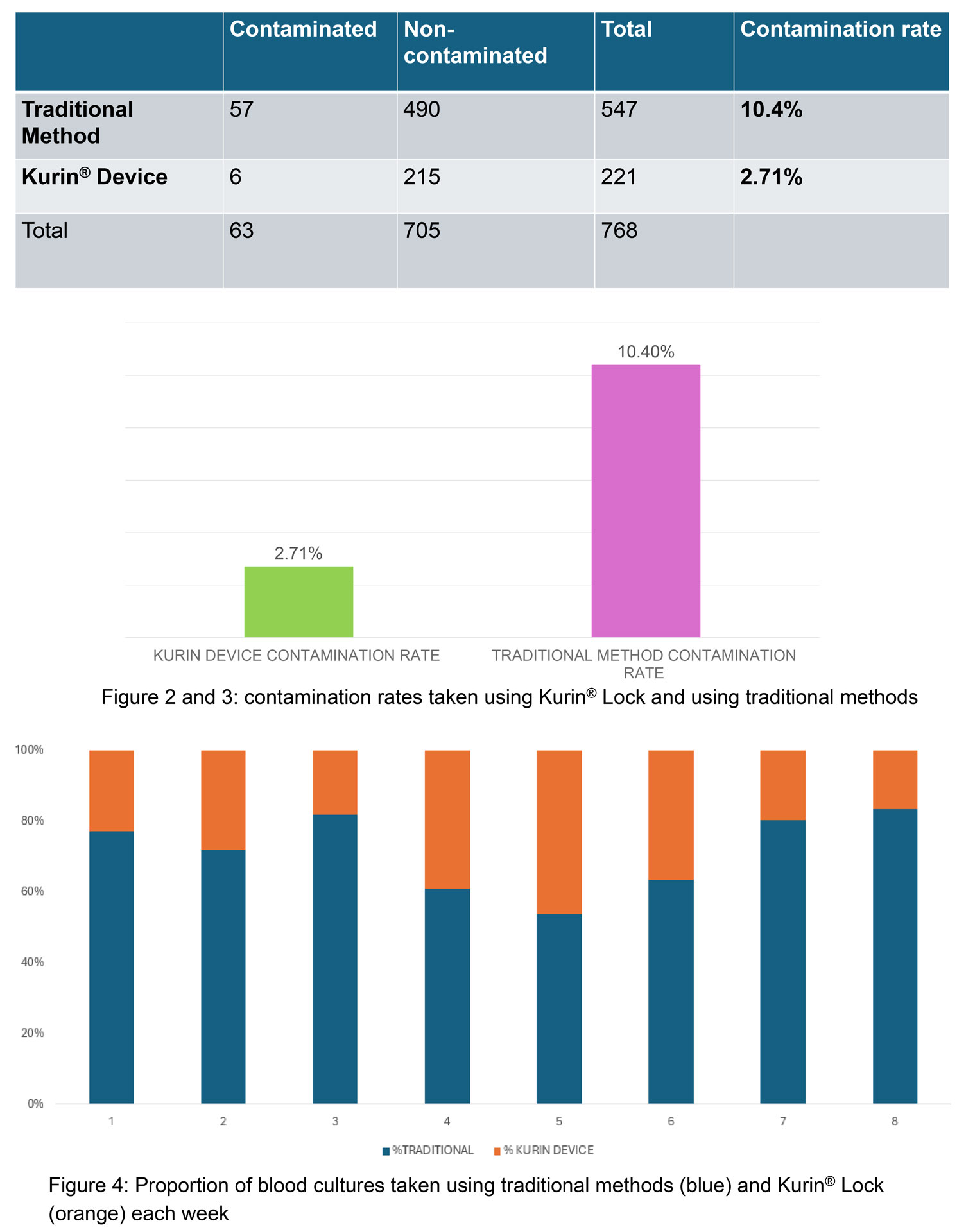
A retrospective study was conducted including all patients with a blood culture collected in two UMass Memorial Health emergency departments from September 2019 through April 2020. Contaminants were identified by standard clinical microbiology lab criteria and by independent retrospective review by 3 infectious disease (ID) physicians and an Infectious Disease fellow.
Results
5,673 blood samples were obtained with 5,661 samples analyzed after 12 were deemed inconclusive by the ID physician review. Blood culture contaminants accounted for 22.5% of the positive blood cultures. Staphylococcus epidermidis was the most frequent contaminant (33.4%) while Escherichia coli was the most frequent pathogen (21%) causing true bacteremia. Coagulasenegative staphylococci remain the most frequent cause of blood culture contamination with Staphylococcus epidermidis being the most common. Staphylococcus auricularis, Staphylococcus caprae, lentus, Staphylococcus pseudointermedius, Staphylococcus saccharolyticus, and Staphylococcus warneri were all determined to be contaminants in 100% of cases.
Relevant Conclusions
The results of the prospective controlled trial associated with this study affirm that the blood diversion device did lead to a decrease in blood culture contamination rates in busy ED in both the intention-to-use and actual-use analyses. “These devices offer a cost-effective and clinically beneficial strategy to improve diagnostic accuracy and patient outcomes. We anticipate that this approach could help combat the issue of increasing antibiotic resistance.”
Read full text here