Reducing the Blood Culture Contamination Rate in the Emergency Department: A Good Start
E Browne, M Russell, G Kalukondanahally, M Abbas, O Keane, and H McDermott
Beaumont Hospital, Dublin, Ireland
Poster number: P1939
Background
Blood culture is the primary diagnostic test available to detect bloodstream infection1, however contamination during collection remains a significant issue
- Contamination complicates clinical interpretation, and can have significant clinical and financial consequences, including unnecessary antimicrobial treatment, increased length of hospital stays, and escalating healthcare costs2,3
- Current guidelines recommend that blood culture contamination rates should not exceed 3%, with a goal of 1% when best practices are followed2,4
- In Q1 of 2024, Beaumont Hospital (Dublin, Ireland) Emergency Department had a median contamination rate of 10%
- Kurin Lock® blood culture collection sets divert the initial 0.15ml of blood drawn (which may contain contaminants from the patient’s skin) into a flash chamber
- The device is easy to use, with minimal training required, and costs approx. £20
- Clinical trial evidence suggests that Kurin Lock® is a safe and effective way of reducing blood culture contamination rates, compared with standard blood culture collection5

Objectives
Aim of the quality improvement project: reduce blood culture contamination rate in Beaumont Hospital ED by implementation of Kurin Lock® device for blood culture collection
Methods
Kurin Lock® introduced on pilot basis (8th May to 2nd July 2024)
↓
130 ED healthcare workers (HCWs) trained in device use
↓
HCWs document blood culture collection
- Complete and sign Kurin® form
- Affix patient label
- Deposit form in collection bucket
↓
Weekly form collection and data entry
- Forms collected and processed
- Data (date and time of blood culture collection, HCW name, patient episode number) entered into Excel
↓
Incentive
- Restaurant voucher awarded for most Kurin® blood cultures collected
↓
Blood culture contamination data sourced from Beaumont surveillance database
↓
Weekly comparison of contamination rates
- Kurin® vs Traditional Method
- Contaminants determined by Clinical Microbiology Team
Results
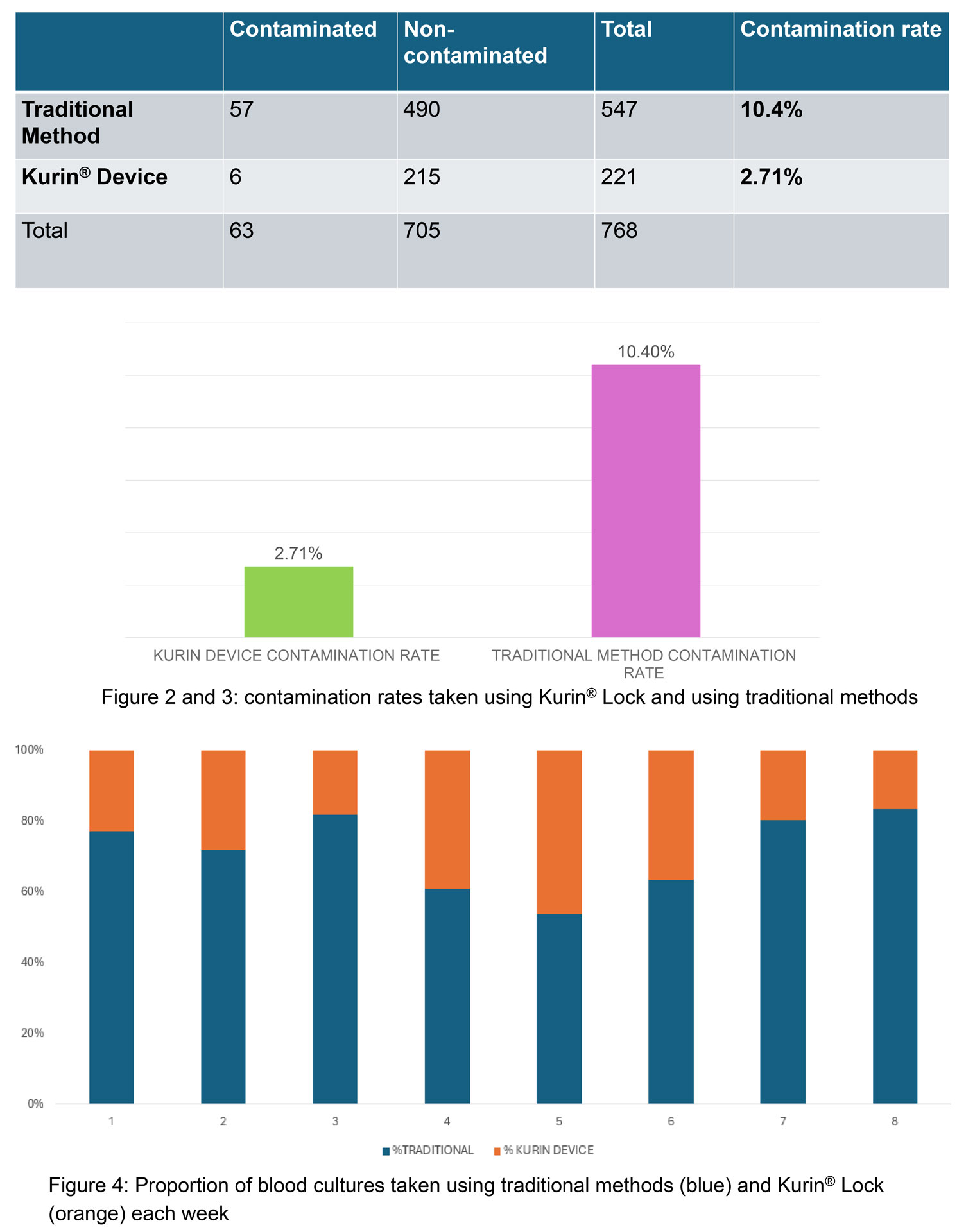
A total of 768 blood cultures were collected in ED over 8 weeks (see figure 2)
- The contamination rate was significantly lower with the Kurin® Lock (2.71%) compared to the traditional method (10.4%) (χ² = 12.41, df=1, p= 0.0004)
- Staff adherence to using the Kurin® Lock averaged 28.38% (range: 16%-46%) (see figure 4).
Discussion
Implementation of the Kurin® Lock in Beaumont Hospital ED resulted in a significant reduction in blood culture contamination rates compared to the traditional collection method.
- NICE guidelines5(2024) recommend the Kurin® Lock to reduce blood culture contamination in emergency departments
- Following the pilot trial, a business proposal for permanent adoption of the Kurin® Lock was developed and approved, leading to its formal implementation in Beaumont Hospital ED in March 2025.
- Economic modelling suggests a high probability of cost savings when used in EDs with baseline contamination rates >9%, with previous estimates indicating potential savings of ~£5,000 per contaminated blood culture6.
- Despite its benefits, low adherence to Kurin® Lock use among ED HCWs was a key challenge in our pilot trial
- To address this, an ED staff survey and feedback session could identify barriers. Targeted education, training, and incentivisation for ED doctors, nurses, and staff could improve adherence. Involving the phlebotomy and IV cannulation teams in blood culture collection may streamline and standardise the process, maximising impact.
Read full text here
REFERENCES:
1 “Investigation of blood cultures (for organisms other than Mycobacterium species)” (UK Standards for Microbiology Investigations, PHE 2019)
2 Doern, GV et al “Practical Guidance for Clinical Microbiology Laboratories: A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem”. Clinical Microbiology Reviews 2020; Oct 30;33(1).
3 Palavecino, EL et al “Laboratory approaches to determining blood culture contamination rates: an ASM Laboratory Practices Subcommittee report”. Journal of Clinical Microbiology 2024; Feb 14;62(2)
4 CLSI. Principles and Procedures for Blood Cultures. 2nd Ed. CLSI Guideline M47. Clinical and Laboratory Standards Institute; 2022.
5 “Kurin Lock for blood culture collection” (NICE guidance, April 2024)
6 Alahmadi, YM et al “Clinical and economic impact of contaminated blood cultures within the hospital setting”. Journal of Hospital Infection 2011; Mar 77(3):233-6
